Explain Solid Liquid Mixture . a mixture consists of two or more chemically distinct components that do not react with each other. mixtures can be separated using a variety of techniques. For example, water can be separated from salty water by simple distillation. mixtures of solids in liquids can be presented in different ways, according to the characteristics of these components:. a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. Mixtures can be solids, liquids, gases, or a combination of states of matter. Distillation separates a liquid from a solution. know about 12 different methods of separation of mixtures: Chromatography involves solvent separation on a solid medium. the point labeled “e 2 ” is the eutectic point, meaning the composition for which the mixture of the two solids has the lowest melting point. No matter where you sample the. The four main regions can be described as below:
from igcsechemistryrevision.weebly.com
mixtures can be separated using a variety of techniques. mixtures of solids in liquids can be presented in different ways, according to the characteristics of these components:. No matter where you sample the. know about 12 different methods of separation of mixtures: Distillation separates a liquid from a solution. Chromatography involves solvent separation on a solid medium. a mixture consists of two or more chemically distinct components that do not react with each other. the point labeled “e 2 ” is the eutectic point, meaning the composition for which the mixture of the two solids has the lowest melting point. a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. For example, water can be separated from salty water by simple distillation.
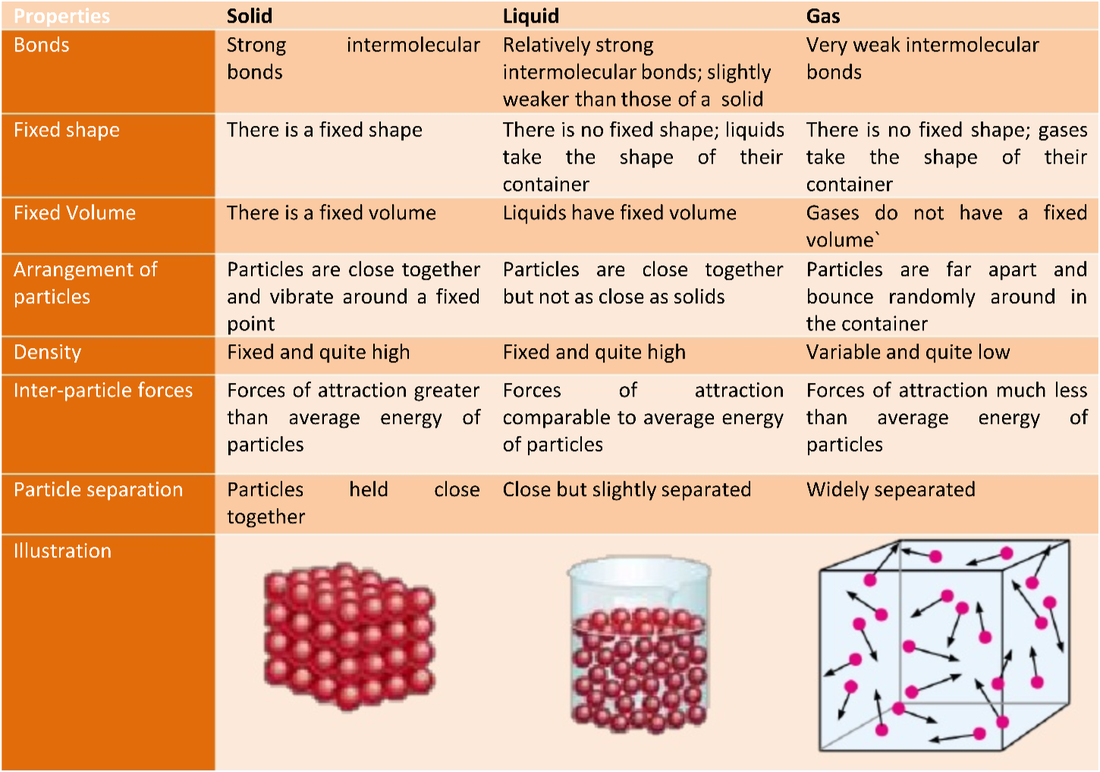
1.1 Understand the arrangement, movement and energy of particles in
Explain Solid Liquid Mixture mixtures of solids in liquids can be presented in different ways, according to the characteristics of these components:. Chromatography involves solvent separation on a solid medium. For example, water can be separated from salty water by simple distillation. mixtures of solids in liquids can be presented in different ways, according to the characteristics of these components:. know about 12 different methods of separation of mixtures: the point labeled “e 2 ” is the eutectic point, meaning the composition for which the mixture of the two solids has the lowest melting point. mixtures can be separated using a variety of techniques. No matter where you sample the. The four main regions can be described as below: Mixtures can be solids, liquids, gases, or a combination of states of matter. Distillation separates a liquid from a solution. a mixture consists of two or more chemically distinct components that do not react with each other. a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition.
From primaryleap.co.uk
Chemistry Solutions And Mixtures Level 1 activity for kids Explain Solid Liquid Mixture Mixtures can be solids, liquids, gases, or a combination of states of matter. mixtures of solids in liquids can be presented in different ways, according to the characteristics of these components:. a mixture consists of two or more chemically distinct components that do not react with each other. Distillation separates a liquid from a solution. For example, water. Explain Solid Liquid Mixture.
From www.youtube.com
SOLID LIQUID MIXTURE YouTube Explain Solid Liquid Mixture The four main regions can be described as below: Distillation separates a liquid from a solution. Mixtures can be solids, liquids, gases, or a combination of states of matter. Chromatography involves solvent separation on a solid medium. For example, water can be separated from salty water by simple distillation. a mixture consists of two or more chemically distinct components. Explain Solid Liquid Mixture.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas Explain Solid Liquid Mixture No matter where you sample the. mixtures can be separated using a variety of techniques. mixtures of solids in liquids can be presented in different ways, according to the characteristics of these components:. Distillation separates a liquid from a solution. Chromatography involves solvent separation on a solid medium. the point labeled “e 2 ” is the eutectic. Explain Solid Liquid Mixture.
From www.youtube.com
SEPARATION OF SOLID LIQUID MIXTURECLASS 7 YouTube Explain Solid Liquid Mixture know about 12 different methods of separation of mixtures: mixtures of solids in liquids can be presented in different ways, according to the characteristics of these components:. Distillation separates a liquid from a solution. Mixtures can be solids, liquids, gases, or a combination of states of matter. mixtures can be separated using a variety of techniques. . Explain Solid Liquid Mixture.
From mungfali.com
10 Examples Of Mixtures Explain Solid Liquid Mixture the point labeled “e 2 ” is the eutectic point, meaning the composition for which the mixture of the two solids has the lowest melting point. know about 12 different methods of separation of mixtures: Distillation separates a liquid from a solution. a mixture consists of two or more chemically distinct components that do not react with. Explain Solid Liquid Mixture.
From dxodcynea.blob.core.windows.net
For Solid Liquid Mixture at Janell Levitt blog Explain Solid Liquid Mixture a mixture consists of two or more chemically distinct components that do not react with each other. a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. For example, water can be separated from salty water by simple distillation. know about 12 different methods of separation of mixtures: Chromatography involves solvent separation. Explain Solid Liquid Mixture.
From schematicdiagrampoukes.z13.web.core.windows.net
Diagram Of Mixture Explain Solid Liquid Mixture a mixture consists of two or more chemically distinct components that do not react with each other. The four main regions can be described as below: Mixtures can be solids, liquids, gases, or a combination of states of matter. know about 12 different methods of separation of mixtures: Distillation separates a liquid from a solution. mixtures of. Explain Solid Liquid Mixture.
From sciencemsqblog8.blogspot.com
Science8 Semester 2,Chapter 4 Mixtures Explain Solid Liquid Mixture Mixtures can be solids, liquids, gases, or a combination of states of matter. mixtures of solids in liquids can be presented in different ways, according to the characteristics of these components:. a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. Distillation separates a liquid from a solution. mixtures can be separated. Explain Solid Liquid Mixture.
From sciencetallis.weebly.com
3. Particle Model of Matter THOMAS TALLIS SCIENCE Explain Solid Liquid Mixture the point labeled “e 2 ” is the eutectic point, meaning the composition for which the mixture of the two solids has the lowest melting point. For example, water can be separated from salty water by simple distillation. mixtures can be separated using a variety of techniques. The four main regions can be described as below: Chromatography involves. Explain Solid Liquid Mixture.
From animalia-life.club
Mixtures Examples Explain Solid Liquid Mixture the point labeled “e 2 ” is the eutectic point, meaning the composition for which the mixture of the two solids has the lowest melting point. mixtures can be separated using a variety of techniques. a mixture consists of two or more chemically distinct components that do not react with each other. The four main regions can. Explain Solid Liquid Mixture.
From exoipbldu.blob.core.windows.net
A Solid Is Stirred Into A Liquid And Dissolves. Which Type Of Mixture Explain Solid Liquid Mixture a mixture consists of two or more chemically distinct components that do not react with each other. a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. know about 12 different methods of separation of mixtures: The four main regions can be described as below: mixtures of solids in liquids can. Explain Solid Liquid Mixture.
From dxodcynea.blob.core.windows.net
For Solid Liquid Mixture at Janell Levitt blog Explain Solid Liquid Mixture For example, water can be separated from salty water by simple distillation. Distillation separates a liquid from a solution. Mixtures can be solids, liquids, gases, or a combination of states of matter. know about 12 different methods of separation of mixtures: mixtures can be separated using a variety of techniques. Chromatography involves solvent separation on a solid medium.. Explain Solid Liquid Mixture.
From www.youtube.com
Upper Sec IP Chem Solid Liquid Mixtures Filtration, Evaporation Explain Solid Liquid Mixture Chromatography involves solvent separation on a solid medium. a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. a mixture consists of two or more chemically distinct components that do not react with each other. Distillation separates a liquid from a solution. mixtures of solids in liquids can be presented in different. Explain Solid Liquid Mixture.
From fr.dreamstime.com
Illustration Pour Des Changements D'état Entre Solide, Le Liquide Et Le Explain Solid Liquid Mixture No matter where you sample the. Mixtures can be solids, liquids, gases, or a combination of states of matter. mixtures can be separated using a variety of techniques. Distillation separates a liquid from a solution. For example, water can be separated from salty water by simple distillation. mixtures of solids in liquids can be presented in different ways,. Explain Solid Liquid Mixture.
From courses.lumenlearning.com
The Dissolution Process Chemistry Explain Solid Liquid Mixture a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. the point labeled “e 2 ” is the eutectic point, meaning the composition for which the mixture of the two solids has the lowest melting point. mixtures can be separated using a variety of techniques. mixtures of solids in liquids can. Explain Solid Liquid Mixture.
From www.youtube.com
DIY Solid Liquid Mixture YouTube Explain Solid Liquid Mixture The four main regions can be described as below: mixtures of solids in liquids can be presented in different ways, according to the characteristics of these components:. Chromatography involves solvent separation on a solid medium. mixtures can be separated using a variety of techniques. For example, water can be separated from salty water by simple distillation. No matter. Explain Solid Liquid Mixture.
From www.dreamstime.com
Solution. Solid in liquid stock vector. Illustration of expansion Explain Solid Liquid Mixture mixtures can be separated using a variety of techniques. the point labeled “e 2 ” is the eutectic point, meaning the composition for which the mixture of the two solids has the lowest melting point. Chromatography involves solvent separation on a solid medium. a mixture consists of two or more chemically distinct components that do not react. Explain Solid Liquid Mixture.
From www.pinterest.com
Mixture Easy Science Easy science, Flashcards, Mixtures Explain Solid Liquid Mixture The four main regions can be described as below: a homogeneous mixture is a solid, liquid, or gaseous mixture that has a uniform composition. Distillation separates a liquid from a solution. No matter where you sample the. mixtures can be separated using a variety of techniques. For example, water can be separated from salty water by simple distillation.. Explain Solid Liquid Mixture.